Radioactive isotopes create the majority of Earth’s heat. To understand this radioactive origin of geothermal energy, we must examine nuclides.
Nuclides and Isotopes
All atoms have protons, neutrons, and electrons. A nuclide is a term used to characterize atoms by their number of protons, their number of neutrons, and their nuclear energy state. Isotopes describe nuclides in greater detail; they are nuclides of a particular element that differ from one another based on their number of neutrons. So, when a scientist uses the word, “isotopes,” they are referring to a set of nuclides with the same number of protons, but different numbers of neutrons.
For example, let’s take a look at the element, carbon. The figure below illustrates the three common carbon isotopes, distinguished by the differences in the number of neutrons (brown-filled circles) in the nucleus: carbon-12 (12C), carbon-13 (13C), and carbon-14 (14C).
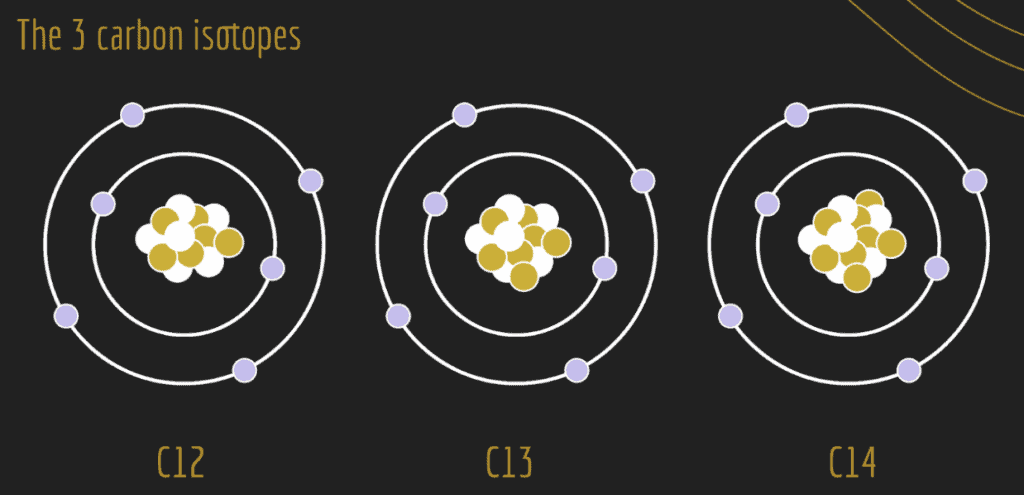
Stable vs. Unstable Isotopes
Some isotopes are considered stable and others are considered unstable. Instability occurs as a result of an imbalance in the number of protons and neutrons in the atomic nucleus. The greater the imbalance, the more likely that an isotope will be unstable. For example, 12C and 13C are stable, whereas 14C is unstable. An unstable isotope has excess internal energy. The unstable isotope can shift to a more stable configuration by giving up some of its energy. The unstable isotope achieves a more stable form by emitting energetic particles. This process is called radioactive decay and the unstable isotopes emit radiation (energy in the form of electromagnetic waves) and are called radioisotopes.
Carbon Isotope Examples and Radioactive Decay
Considering the element carbon, 12C and 13C are stable isotopes and therefore are not considered radioactive. However, 14C is radioactive (unstable) due to the extra weight of its two additional neutrons, and therefore, experiences radioactive decay. The radioactive decay emits radiation and produces heat as the parent isotope decays into a stable form: the daughter isotope. With carbon, the radioactive parent isotope 14C will decay to the stable daughter isotope 14N. During this process, one of the eight neutrons in the 14C nuclide becomes a proton, decreasing the number of neutrons to seven and increasing the number of protons from six to seven. This radioactive decay has created the stable daughter isotope of nitrogen: 14N.
Some examples of parent and daughter isotopes are:
| Parent isotope (unstable) | Daughter isotope (stable) |
|---|---|
| carbon-14 | nitrogen-14 |
| uranium-238 | lead-206 |
| potassium-40 | calcium-40 |
Radiogenic Heating
As previously mentioned, unstable isotopes become more stable by releasing energy during radioactive decay. The release of heat energy from radioactive decay is known as radiogenic heating. On Earth, four radioactive isotopes are responsible for the majority of radiogenic heat because of their enrichment relative to other radioactive isotopes: uranium-238 (238U), uranium-235 (235U), thorium-232 (232Th), and potassium-40 (40K).
Movement of Heat Through the Earth
As heat is released within the core, that energy moves. Temperatures at the core-mantle boundary can reach over 4,000 °C (7,200 °F).1Lay, T., Hernlund, J., & Buffett, B. A. (2008). Core–mantle boundary heat flow. Nature geoscience, 1(1), 25-32. The core’s heat creates a convection current within the mantle layer that, not only assists in heating the crust to create geothermal energy, but also, moves Earth’s tectonic plates. The core heats magma causing the magma to rise towards the crust; while the magma closer to the crust cools and drops towards the core. The rising magma heats aquifers and rocks, creating steam vents, hot springs, geysers, underwater hydrothermal vents, and other features essential for harnessing geothermal energy.2National Geographic. (n.d.). Geothermal Energy. National Geographic Education. Although we frequently observe the movement of steam, water, or magma, most of Earth’s geothermal energy is dry and at depth. Many accumulations of high heat within Earth’s crust can only be accessed by drilling to the appropriate depth.
True or false – The instability of a radioactive isotope originates from the addition of neutrons to a stable nuclide.
True
Correct
False
Incorrect
Most of Earth’s geothermal energy is __________________.
Wet
Incorrect
Dry
Correct
Which one of these isotopes is not responsible for the majority of radiogenic heat in the earth?
thorium-232
Incorrect
carbon-14
Correct
uranium-238
Incorrect
uranium-235
Incorrect
Image Credits
- 3 Carbon Isotopes: Gwen Olivier